Issued on behalf of Oncolytics Biotech Inc.
VANCOUVER – Baystreet.ca News Commentary – More and more young women are getting diagnosed with cancer than ever before, causing many to sound the alarm about reduced funding for prevention and treatment. Proposed budget cuts on the horizon for funding scientific research could put the development of new cancer cures in jeopardy. As well, there is an atmosphere of “tremendous uncertainty” for cancer research as US regulators have begun to re-examine mRNA vaccines at both the federal and state levels. On the ground, biotechs in the oncology space are emerging with potential milestones in the near future that may shift the tide in the sector, including Oncolytics Biotech Inc. (NASDAQ: ONCY) (TSX: ONC), Citius Oncology, Inc. (NASDAQ: CTOR), Citius Pharmaceuticals, Inc. (NASDAQ: CTXR), RadNet, Inc. (NASDAQ: RDNT), and iCAD, Inc. (NASDAQ: ICAD).
Colorectal and other GI cancers are rising fastest among younger adults, adding urgency for fresh oncology options even as policy hurdles slow research. Market watchers now see the global cancer‑drug sector topping US$900billion in sales by 2034. Precedence Research pegs the next‑generation therapeutics slice—powered by precision and personalized medicine—at US$175.2billion that same year, growing at a 7.35% CAGR.
Oncolytics Biotech Inc. (NASDAQ: ONCY) (TSX: ONC) recently held a well‑attended key opinion leader (KOL) webinar where pancreatic and gastrointestinal cancer specialists dissected pelareorep’s clinical history, its biomarker data package, and where the oncolytic virus could slot into evolving chemotherapy and immunotherapy regimens.
“I want to thank our esteemed panel of oncologists for their meaningful contributions to our KOL event,” said Jared Kelly, CEO of Oncolytics. “Their insights underscore a critical and timely message: pelareorep is a compelling immunotherapy platform that is well situated for combination strategies and a highly differentiated asset for pharma partners looking to expand or establish leadership in GI oncology. We are committed to moving forward aggressively toward registrational development while engaging with partners who share our vision of transforming outcomes in these difficult-to-treat cancers.”
The panel revisited survival gains in metastatic pancreatic ductal adenocarcinoma (mPDAC), reviewed translational evidence of “cold‑to‑hot” tumor conversion, and outlined next steps for a registration-enabling study. Management said feedback from the discussion would inform both upcoming trial designs and partnering talks now underway.
The event came on the heels of Oncolytics having rolled out an expanded translational‑data review that tightened the scientific case for pelareorep, an intravenously delivered oncolytic reovirus. Renewed analyses from the GOBLET gastrointestinal cancer study and the AWARE‑1 breast cancer study confirmed that the virus replicates inside tumors, switches on interferon signaling in the immune system, and draws tumor‑infiltrating lymphocytes into the tumor microenvironment.
“This robust data set, amassed from several studies in cancers that have historically resisted immunotherapeutic approaches, provides definitive validation of pelareorep’s immune-mediated mechanism of action,” said Dr. Thomas Heineman, Chief Medical Officer of Oncolytics. “We observed tumor biopsy-confirmed virus replication, immune cell activation, and the recruitment of cytotoxic T cells into the TME – all consistent with the durable responses observed in patients with metastatic PDAC and HR+/HER2- breast cancer who were treated with pelareorep.”
Investigators also recorded higher PD‑L1 expression and tracked newly expanded cytotoxic T‑cell clones in blood samples that matched those inside shrinking lesions—molecular fingerprints that point to stronger responses when pelareorep is combined with standard-of-care treatments and checkpoint inhibitors.
“The collection of data here show that pelareorep works how a cancer immunotherapy should work,” said Jared Kelly, CEO of Oncolytics. “Pelareorep is a versatile product candidate with strong platform potential to enhance immunological responses in multiple indications, including hard-to-treat cancers. Such compelling findings should be exciting to strategic partners focused on finding a platform immunotherapy in large indications with high unmet medical needs.”
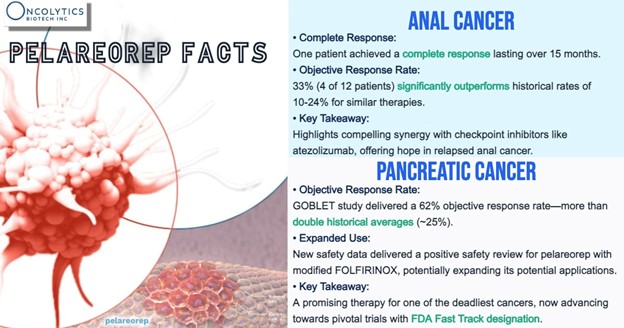
Clinical outcomes already hint at real‑world benefit. In more than 100 first‑line mPDAC patients, pelareorep‑based regimens achieved a 21.9% two‑year overall‑survival rate versus a 9.2% historical benchmark. A separate single‑arm study that paired pelareorep with chemotherapy and a checkpoint blocker produced a 62% objective‑response rate—especially notable given that no checkpoint therapy is approved in this cancer today.
In hormone‑receptor‑positive, HER2‑negative metastatic breast cancer (HR+/HER2‑ mBC), two randomized trials added more than ten months of median overall survival, while BRACELET‑1 nearly doubled median progression‑free survival to 12.1 months compared with 6.4 months for control patients.
To steer these data toward value‑creating deals and late‑stage trials, the company strengthened its leadership earlier this year by appointing industry veteran Jared Kelly as CEO and naming Andrew Aromando Chief Business Officer. The duo previously helped guide Ambrx Biopharma into a US$2 billion sale to Johnson & Johnson, experience the board believes will support capital‑efficient development and strategic partnering for pelareorep.
“Pelareorep’s clinical data across multiple tumors is striking and represents the potential for a true backbone immunotherapy to address many in-need indications,” said Kelly. “With a renewed focus and sharpened clinical development plan, we believe we will move pelareorep forward effectively and efficiently to a place where potential partners will see the value of a de-risked immunotherapy.”
As CBO, Aromando is now leading global business development and helping shape the company’s corporate, clinical, and regulatory strategies. The leadership tandem is expected to prioritize partnering and expansion opportunities while preserving capital efficiency—a strategy well-suited for pelareorep’s growing clinical profile.
“I’m thrilled to join Oncolytics at such a pivotal moment in its evolution,” said Aromando. “With promising data in difficult-to-treat cancers and a compelling body of clinical evidence in over 1,100 patients, I believe the Company is uniquely positioned to deliver meaningful value to patients and other stakeholders in the near term.”
Pelareorep currently holds FDA Fast Track designations in mPDAC (pancreatic cancer) and HR+/HER2‑ mBC (breast cancer), along with Orphan‑Drug status for pancreatic cancer in the United States and Europe.
With mechanistic proof, survival data that outperform historical controls, and fresh validation from the recent KOL event, Oncolytics Biotech is aligning its clinical, regulatory, and business strategies to move pelareorep into registration‑enabling trials and position it as a backbone immunotherapy across solid tumors.
CONTINUED… Read this and more news for Oncolytics Biotech at: https://usanewsgroup.com/2023/10/02/the-most-undervalued-oncolytics-company-on-the-nasdaq/
In other recent industry developments and happenings in the market include:
Citius Oncology, Inc. (NASDAQ: CTOR) and its 92%‑owned Citius Pharmaceuticals, Inc. (NASDAQ: CTXR) recently signed a distribution‑services agreement with Cencora that widens U.S. access to LYMPHIR, the FDA‑approved immunotherapy for relapsed or refractory cutaneous T‑cell lymphoma.
"As we move closer to the U.S. market introduction of LYMPHIR, we remain focused on disciplined execution across all key commercial readiness activities," said Leonard Mazur, Chairman and CEO of Citius Oncology and Citius Pharmaceuticals. "Our agreement with Cencora adds further depth to our distribution strategy and strengthens our ability to deliver LYMPHIR to treatment centers across the country. These foundational partnerships demonstrate our ongoing commitment to building a launch platform that supports near-term revenue and long-term shareholder value."
The wholesale deal positions Cencora to supply treatment centers nationwide once commercial shipments begin. Management says the launch targets an underserved US$400 million CTCL market and stresses “disciplined execution” on logistics to capture early revenue. LYMPHIR, a fusion protein that binds IL‑2 receptors causing diphtheria toxin fragments to inhibit protein synthesis, received FDA approval in August 2024 and has prior clearance in Japan. The Cencora partnership scales the distribution network and reinforces long‑term growth plans for both companies.
RadNet, Inc. (NASDAQ: RDNT) recently announced that its digital‑health arm DeepHealth has completed the acquisition of iCAD, Inc. (NASDAQ: ICAD), uniting two AI breast‑imaging leaders under one roof. The merger creates an end‑to‑end platform capable of reading more than 10 million mammograms a year while improving detection accuracy and workflow efficiency.
“We are excited to welcome the iCAD team to DeepHealth,” said Kees Wesdorp, President and CEO of RadNet’s Digital Health segment. “The integration of iCAD further empowers DeepHealth to meet the real-world clinical needs of today, from improving the accuracy and early detection of breast cancer to orchestrating large-scale screening programs, while shaping the future of breast cancer management. Our combined capabilities position us for accelerated growth and rapid delivery of transformative, AI-powered population health solutions.”
iCAD already serves over 1,500 provider sites in 50 countries, giving DeepHealth immediate international reach. By adding iCAD’s commercial and regulatory expertise, RadNet aims to drive faster revenue growth in the expanding AI‑powered breast‑cancer‑screening market.
Source: https://usanewsgroup.com/2024/09/21/is-oncolytics-biotech-the-markets-most-undervalued-cancer-opportunity/
CONTACT:
Baystreet.ca
[email protected]
(250) 999-4849
DISCLAIMER: Nothing in this publication should be considered as personalized financial advice. We are not licensed under securities laws to address your particular financial situation. No communication by our employees to you should be deemed as personalized financial advice. Please consult a licensed financial advisor before making any investment decision. This is a paid advertisement and is neither an offer nor recommendation to buy or sell any security. We hold no investment licenses and are thus neither licensed nor qualified to provide investment advice. The content in this report or email is not provided to any individual with a view toward their individual circumstances. Baystreet.ca is a wholly-owned subsidiary of Baystreet.ca Media Corp. (“BAY”) BAY has been not been paid a fee for Oncolytics Biotech Inc. advertising and/or digital media, but the owner(s) of BAY also own Market IQ Media Group, Inc., which has been paid a fee from the company directly. There may be 3rd parties who may have shares Oncolytics Biotech Inc., and may liquidate their shares which could have a negative effect on the price of the stock. This compensation constitutes a conflict of interest as to our ability to remain objective in our communication regarding the profiled company. Because of this conflict, individuals are strongly encouraged to not use this publication as the basis for any investment decision. The owner/operator of BAY own shares of Oncolytics Biotech Inc. which were purchased in the open market. BAY and all of it’s respective employees, owners and affiliates reserve the right to buy and sell, and will buy and sell shares of Oncolytics Biotech Inc. at any time thereafter without any further notice. We also expect further compensation as an ongoing digital media effort to increase visibility for the company, no further notice will be given, but let this disclaimer serve as notice that all material disseminated by BAY has been approved by the above mentioned company; this is a paid advertisement, and we own shares of the mentioned company that we will sell, and we also reserve the right to buy shares of the company in the open market, or through other investment vehicles. While all information is believed to be reliable, it is not guaranteed by us to be accurate. Individuals should assume that all information contained in our newsletter is not trustworthy unless verified by their own independent research. Also, because events and circumstances frequently do not occur as expected, there will likely be differences between any predictions and actual results. Always consult a licensed investment professional before making any investment decision. Be extremely careful, investing in securities carries a high degree of risk; you may likely lose some or all of the investment.