Issued on behalf of Oncolytics Biotech
Inc.
VANCOUVER – USA News Group News Commentary – One of the largest and most advanced cancer research and treatment organizations in the USA, City of Hope, predicts 2025 will be a big year in cancer advances. At the end of 2024, experts were projecting several developments in oncology, addressing the concern of rising rates of multiple types of cancer. The American Association for Cancer Research (AACR) has stated it looks forward to advancements across precision medicine, immunotherapy, hematologic malignancies, artificial intelligence, and cancer disparities and prevention. Behind the scenes, several biotech innovators have been making solid advancements, including developments from Oncolytics Biotech Inc. (NASDAQ: ONCY) (TSX: ONC), GRAIL, Inc. (NASADAQ: GRAL), Guardant Health, Inc. (NASDAQ: GH), Personalis, Inc. (NASDAQ: PSNL), and Legend Biotech Corporation (NASDAQ: LEGN).
The article continued: DelveInsight Business Research projected that the Global Cancer Therapy Market is expected to grow at a 9.12% CAGR to reach US$285.96 billion by 2030. The Center for Innovation and Translation of Point of Care Technologies for Equitable Cancer Care (CITEC) recently announced a global collaboration that funds new projects to advance cancer detection technologies.
Oncolytics Biotech Inc. (NASDAQ: ONCY) (TSX: ONC), a leading clinical-stage company specializing in immunotherapy for oncology, today shared details about the data it will present at the ASCO GI symposium. The presentations will highlight pelareorep, an innovative immunotherapy that trains the immune system to target cancer by turning “cold” tumors—typically resistant to treatment—into “hot” tumors that respond better to therapy.
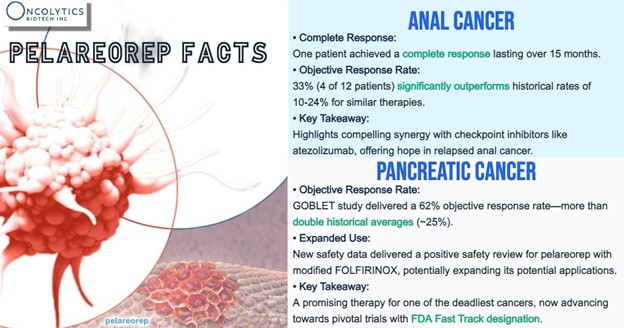
In relapsed anal cancer, 4 out of 12 evaluable patients achieved a partial response for a response rate of 33%, and one patient achieved a remarkable complete response, meaning their cancer became undetectable and remained so for over 15 months. To put this into perspective, similar treatments typically achieve response rates of only 10-24%. This underscores pelareorep’s potential to deliver life-changing results in some of the toughest-to-treat cancers.
“In relapsed anal cancer, the efficacy signal that was initially reported continues to outperform historical control trials with the inclusion of additional patients,” said Thomas Heineman, M.D., Ph.D., Chief Medical Officer for Oncolytics Biotech. “Importantly, the complete response we observed previously continued beyond the 12 months initially reported. Together, these results point to a clinically meaningful synergy between pelareorep and checkpoint inhibitors like atezolizumab.”
In pancreatic cancer, pelareorep has also demonstrated strong potential to improve outcomes for patients with this aggressive disease. The upcoming ASCO GI presentation will feature new safety data showing that pelareorep can be combined with modified FOLFIRINOX, another widely used chemotherapy regimen for patients with pancreatic cancer.
“Our new safety data indicate its ability to also be combined with modified FOLFIRINOX, thus expanding its potential to benefit patients with metastatic pancreatic cancer,” added Dr. Heineman. “We will continue to provide updates on the safety and efficacy of pelareorep-based combination therapy from these cohorts as they become available.”
This builds on prior results from the GOBLET study, where pelareorep, combined with atezolizumab, gemcitabine, and nab-paclitaxel, achieved a 62% objective response rate—more than double historical averages of 25%. These findings were pivotal in earning pelareorep FDA Fast Track designation in 2022, highlighting its promise to fill the critical unmet need for more effective pancreatic cancer therapies.
The ability to pair pelareorep with modified FOLFIRINOX represents an important step forward in expanding treatment options for metastatic pancreatic cancer. These results not only highlight pelareorep’s versatility but also its potential to enhance outcomes across multiple standard-of-care therapies.
“I am quite pleased by these recent updates from the GOBLET study as they continue to provide potential new treatment options for patients in need of alternatives while maintaining a manageable safety profile,” said Dirk Arnold, M.D., Ph.D., Director of Asklepios Tumorzentrum Hamburg, and primary investigator of the GOBLET trial. “I’ve been especially impressed with the ability of pelareorep-based therapies to work across multiple challenging cancer indications and with multiple standards of care, including chemotherapy and checkpoint inhibitors, so I look forward to additional data readouts that can help improve the treatment paradigm.”
As Oncolytics Biotech advances its clinical programs and prepares for pivotal studies, the growing body of evidence continues to solidify pelareorep as a transformative therapeutic option for patients in desperate need of effective treatments.
CONTINUED… Read this and more news for Oncolytics Biotech at: https://usanewsgroup.com/2023/10/02/the-most-undervalued-oncolytics-company-on-the-nasdaq/
In other recent industry developments and happenings in the market include:
GRAIL, Inc. (NASADAQ: GRAL), a healthcare company focused on detecting cancer early, recently published findings from its PATHFINDER study in Lancet Oncology. The study assessed how patients felt about multi-cancer early detection (MCED) testing over 12 months, including their anxiety, distress, quality of life, satisfaction with the Galleri® test, and willingness to continue screenings. Researchers used several tools to measure patient-reported outcomes, revealing important insights into the emotional and practical impacts of early cancer detection testing.
"As the pioneer in multi-cancer early detection, GRAIL is committed to not only evaluating the performance of the Galleri test but also the impact of MCED screening on patients," said Eric Klein, MD, Distinguished Scientist at GRAIL and co-author on the study. "Collecting participant perspectives and appropriately supporting recipients of cancer screening may improve adherence rates and early detection. The patient-reported outcome findings from the PATHFINDER study suggest MCED testing is associated with a high level of satisfaction."
Guardant Health, Inc. (NASDAQ: GH), a leading precision oncology company, recently announced that Medicare has expanded coverage for its Guardant Reveal blood test, which helps monitor colorectal cancer recurrence after treatment. The test detects tiny traces of tumor DNA in the blood, providing a simple, tissue-free way to predict if cancer might return, guiding doctors in their decisions after surgery or chemotherapy. This expansion makes the test available for ongoing colorectal cancer monitoring, addressing a critical gap, as most cancer patients in the U.S. don’t have access to minimal residual disease (MRD) testing. With millions of patients lacking tissue samples for traditional methods, Guardant Reveal offers a convenient and innovative solution.
"Utilizing ctDNA testing in the surveillance setting alongside standard of care monitoring, such as CT scans and CEA testing, has the potential to identify molecular recurrence of colorectal cancer ahead of traditional imaging," said Helmy Eltoukhy, Guardant Health chairman and co-CEO. "This important step by Medicare will make this testing more widely available to patients and support oncologists in making more informed therapeutic decisions."
Personalis, Inc. (NASDAQ: PSNL), working with researchers from London’s Francis Crick Institute and University College London, recently published groundbreaking results in Nature Medicine from the TRACERx lung cancer study. Their NeXT Personal® test, a highly sensitive blood test designed to detect tiny traces of cancer DNA (ctDNA), showed remarkable success in identifying early-stage non-small cell lung cancer (NSCLC). The test detected 100% of non-adenocarcinomas and 81% of adenocarcinomas, a previously hard-to-detect subtype. Importantly, patients with no detectable ctDNA before surgery had a 100% five-year survival rate, while those with ctDNA showed a higher risk of relapse. These findings highlight NeXT Personal’s potential to improve early detection and guide treatment decisions for lung cancer, one of the deadliest cancers in the U.S.
"We designed NeXT Personal to detect residual or recurrent cancer in its earliest stages, and this study shows the clinical importance of that ultra-sensitive detection in early-stage lung cancer," said Richard Chen, MD, MS, Chief Medical Officer and Executive Vice President of R&D at Personalis. "We look forward to continuing our work with the TRACERx team on the broader clinical performance of ctDNA testing in early stage lung cancer. We expect the subsequent publication of those results will help support our submission for Medicare coverage of NeXT Personal Dx in lung cancer."
Legend Biotech Corporation (NASDAQ: LEGN), a global leader in cell therapy, recently announced impressive results from the Phase 3 CARTITUDE-4 study showing that a single infusion of CARVYKTI® significantly improved outcomes for patients with relapsed or refractory multiple myeloma. Patients treated with CARVYKTI® achieved a much higher rate of minimal residual disease (MRD) negativity—89% versus 38% with standard therapies—indicating a strong potential for prolonged survival. The study also found that earlier use of CARVYKTI® in treatment led to better results, supporting its FDA and European approval as the first BCMA-targeted CAR-T cell therapy for multiple myeloma patients. To date, CARVYKTI® has been commercially available in five countries and used by over 4,500 patients, offering new hope for those battling this difficult-to-treat cancer.
“The latest MRD data showcases the advances of CARVYKTI and further demonstrates why it is a leading treatment for patients with multiple myeloma,” said Ying Huang, Ph. D., CEO of Legend Biotech. “As we strive to transform the therapeutic landscape in cancer and beyond, we are proud of the progress made and will continue our efforts to improve the quality of life for those battling incurable diseases.”
Source: https://usanewsgroup.com/2024/09/21/is-oncolytics-biotech-the-markets-most-undervalued-cancer-opportunity/
CONTACT:
USA NEWS GROUP
[email protected]
(604) 265-2873
DISCLAIMER: Nothing in this publication should be considered as personalized financial advice. We are not licensed under securities laws to address your particular financial situation. No communication by our employees to you should be deemed as personalized financial advice. Please consult a licensed financial advisor before making any investment decision. This is a paid advertisement and is neither an offer nor recommendation to buy or sell any security. We hold no investment licenses and are thus neither licensed nor qualified to provide investment advice. The content in this report or email is not provided to any individual with a view toward their individual circumstances. USA News Group is a wholly-owned subsidiary of Market IQ Media Group, Inc. (“MIQ”). MIQ has been paid a fee for Oncolytics Biotech Inc. advertising and digital media from the company directly. There may be 3rd parties who may have shares of Oncolytics Biotech Inc., and may liquidate their shares which could have a negative effect on the price of the stock. This compensation constitutes a conflict of interest as to our ability to remain objective in our communication regarding the profiled company. Because of this conflict, individuals are strongly encouraged to not use this publication as the basis for any investment decision. The owner/operator of MIQ own shares of Oncolytics Biotech Inc. which were purchased in the open market, and reserve the right to buy and sell, and will buy and sell shares of Oncolytics Biotech Inc. at any time without any further notice commencing immediately and ongoing. We also expect further compensation as an ongoing digital media effort to increase visibility for the company, no further notice will be given, but let this disclaimer serve as notice that all material, including this article, which is disseminated by MIQ has been approved by Oncolytics Biotech Inc.; this is a paid advertisement, we currently own shares of Oncolytics Biotech Inc. and will buy and sell shares of the company in the open market, or through private placements, and/or other investment vehicles.
While all information is believed to be reliable, it is not guaranteed by us to be accurate. Individuals should assume that all information contained in our newsletter is not trustworthy unless verified by their own independent research. Also, because events and circumstances frequently do not occur as expected, there will likely be differences between the any predictions and actual results. Always consult a licensed investment professional before making any investment decision. Be extremely careful, investing in securities carries a high degree of risk; you may likely lose some or all of the investment.