Issued on behalf of Oncolytics Biotech Inc.
VANCOUVER – Baystreet.ca News Commentary – As cancer rates continue to climb worldwide, the race to develop better, faster, and more affordable treatments has never been more urgent. Industry forecasters now project the global oncology drug market could top US$900 billion by 2034, driven by heightened demand for precision diagnostics and immune-based therapies. But this explosive growth in market potential comes at a time when the U.S. public health infrastructure may be losing ground. Proposed budget cuts could slash funding for the National Cancer Institute by as much as 40%, raising alarms about future support for federally backed research. With drug costs already drawing scrutiny, much of the innovation burden appears to be shifting toward smaller, agile biotechs — including Oncolytics Biotech Inc. (NASDAQ: ONCY) (TSX: ONC), Agenus Inc. (NASDAQ: AGEN), Olema Pharmaceuticals, Inc. (NASDAQ: OLMA), Actinium Pharmaceuticals, Inc. (NYSE-American: ATNM), and IceCure Medical Ltd. (NASDAQ: ICCM).
This isn’t just a funding gap — it’s a structural shift in how cancer breakthroughs reach the market. As public systems slow, agile biotechs are stepping in with targeted therapies, novel combinations, and faster regulatory engagement. For investors, it’s a key moment to back the next wave of oncology innovation.
Oncolytics Biotech Inc. (NASDAQ: ONCY) (TSX: ONC) has entered a new chapter with the appointment of Jared Kelly as Chief Executive Officer and member of the Board. With a background in high-value biotech transactions and late-stage development strategy, Kelly brings experience that may help position the company for its next phase of clinical and corporate progress.
Prior to joining Oncolytics, Kelly was General Counsel at Ambrx Biopharma, where he played a key role in the company’s $2 billion acquisition by Johnson & Johnson. He also advised a range of life sciences firms on partnerships, licensing, and M&A during his tenure at Kirkland & Ellis LLP and Lowenstein Sandler LLP. His arrival comes as Oncolytics continues advancing pelareorep, a viral-based immunotherapy being evaluated in combination with checkpoint inhibitors and other agents across multiple cancer indications.
“Pelareorep’s clinical data across multiple tumors is striking and represents the potential for a true backbone immunotherapy to address many in-need indications. Importantly, the data show that pelareorep creates a robust immunologic response in difficult tumors and increases survival in a patient population where survival has historically evaded most patients,” said Jared Kelly, CEO of Oncolytics Biotech. “With a renewed focus and sharpened clinical development plan, we believe we will move pelareorep forward effectively and efficiently to a place where potential partners will see the value of a de-risked immunotherapy. I am excited to get to work accelerating development and unlocking significant value for stakeholders.”
Kelly’s appointment appears aligned with a focused strategy: advancing pelareorep through late-stage development while maintaining capital efficiency and openness to potential partnerships. The company’s lead program continues to generate data that support further investigation across several difficult-to-treat cancers.
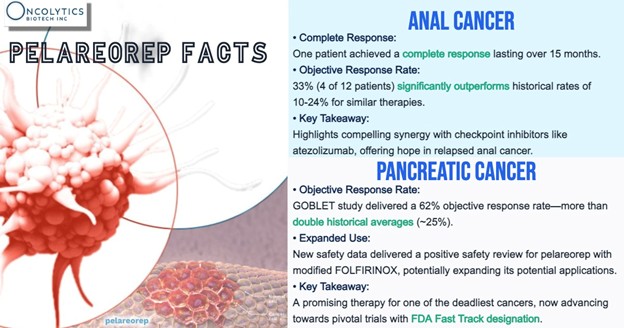
Pelareorep already holds FDA Fast Track designation in two separate indications — metastatic pancreatic ductal adenocarcinoma (mPDAC) and HR+/HER2- metastatic breast cancer (mBC) — a distinction that highlights regulatory interest in its potential.
Across clinical studies, the viral-based immunotherapy has consistently shown signs of immune activation, combinability with checkpoint inhibitors and chemotherapy, and efficacy in heavily pretreated populations.
In mPDAC, a Phase 2 cohort from the trial has reported objective response rates (ORR) above 60% in tumor-evaluable patients, exceeding historical benchmarks for this indication. Additional analyses have noted extended two-year survival rates compared to previous benchmarks. In HR+/HER2- mBC, two randomized Phase 2 trials (IND-213 and BRACELET-1) observed overall survival trends that support continued clinical evaluation.
Elsewhere, a Phase 2 anal cancer cohort combining pelareorep with a checkpoint inhibitor demonstrated partial or complete response rates that exceeded historical control trials for checkpoint inhibitor monotherapy, suggesting potential utility beyond the company’s lead programs.
“Mr. Kelly’s vision and track record is an extraordinary fit with the standout clinical data pelareorep has generated to date,” said Wayne Pisano, Chair of the Board and outgoing Interim CEO of Oncolytics. “We believe Mr. Kelly’s well-documented ability to prioritize clinical program development, execute successful financings, and attract the attention of large industry peers will help maximize Oncolytics’ potential to deliver transformative outcomes for patients and exceptional value for investors.”
Kelly’s compensation framework includes equity-based awards and performance-linked incentives tied to future financings and strategic outcomes. The structure is designed to align leadership priorities with long-term shareholder value while reinforcing a disciplined approach to capital and partnership development.
As multiple cohorts advance within the GOBLET study — including those in pancreatic and anal cancers backed by external funding and regulatory support — Oncolytics appears positioned to continue its progress with a blend of clinical momentum, financial flexibility, and sharpened strategic direction.
Prior to Kelly’s appointment, Oncolytics presented new data from its GOBLET trial at the 2025 ASCO Annual Meeting, highlighting pelareorep’s ability to stimulate both innate and adaptive immune responses in metastatic pancreatic cancer. With fresh clinical insights and new leadership in place, the company appears positioned to advance both its scientific and strategic priorities in tandem.
CONTINUED… Read this and more news for Oncolytics Biotech at: https://usanewsgroup.com/2023/10/02/the-most-undervalued-oncolytics-company-on-the-nasdaq/
In other recent industry developments and happenings in the market include:
Agenus Inc. (NASDAQ: AGEN) has entered a new research collaboration with AI biotech firm Noetik to accelerate development of predictive biomarkers for its lead immunotherapy combo, botensilimab and balstilimab.
"At Agenus, we are committed to transforming cancer care through scientific innovation and next-generation immunotherapies," said Dr. Garo Armen, Chairman and CEO of Agenus. "This collaboration with Noetik enables us to harness cutting-edge AI to better understand patient biology and tailor treatments more precisely. By integrating Noetik’s virtual cell models with our expansive BOT/BAL clinical dataset, we have the potential to accelerate the identification of predictive biomarkers, enhance the success of our pivotal trials, and ultimately improve outcomes for patients who currently have limited or no treatment options."
The partnership leverages Noetik’s OCTO virtual cell model—built on over 200 million tumor and immune cells—to simulate tumor behavior and identify patient response patterns. This AI-enabled approach aims to improve trial outcomes and personalize cancer treatment strategies across multiple solid tumors.
Olema Pharmaceuticals, Inc. (NASDAQ: OLMA) recently announced that 90 mg of palazestrant has been selected as the dose for both its OPERA-01 and OPERA-02 Phase 3 trials in ER+/HER2- metastatic breast cancer.
“We believe the clinical results we have achieved to date for palazestrant across ESR1 mutant and wild-type ER+/HER2- tumors, both in the monotherapy and combination treatment settings, support palazestrant’s potential to have a significant positive impact on breast cancer patients,” said Naseem Zojwalla, M.D., Chief Medical Officer of Olema Oncology. “We remain steadfast in our commitment to these patients, and with 90 mg of palazestrant confirmed as the selected dose, we are focused on rapidly advancing our OPERA-01 and OPERA-02 pivotal trials with top-line data from OPERA-01 anticipated in 2026 and a potential commercial launch in 2027.”
The FDA-aligned decision supports ongoing development as a monotherapy and in combination with ribociclib. Data from OPERA-01 are expected in 2026, with a potential commercial launch in 2027.
Actinium Pharmaceuticals, Inc. (NYSE-American: ATNM) recently presented new data at the Society of Nuclear Medicine & Molecular Imaging (SNMMI) 2025 Annual Meeting showing that its investigational agent ATNM-400 demonstrated superior efficacy in prostate cancer compared to enzalutamide. The data revealed robust tumor reduction in both ARPI-naïve and resistant models and highlighted ATNM-400's ability to overcome resistance to multiple existing treatments.
"We are committed to advancing ATNM-400 to address the high unmet needs that remain in prostate cancer,” said Sandesh Seth, Chairman and CEO of Actinium. “With ATNM-400's target being a disease-driving protein involved in tumor progression and therapeutic resistance combined with the potency and precision of the Ac-225 isotope payload, we believe ATNM-400 has a transformational profile rooted in prostate cancer disease biology, which is strongly supported by our data."
These findings support continued development of ATNM-400 as a differentiated, next-generation radiotherapeutic.
IceCure Medical Ltd. (NASDAQ: ICCM) just secured U.S. patent approval for a new technology that helps control freezing temperatures during cancer treatment using its ProSense® cryoablation system. This system destroys tumors by freezing them, and the new tech helps make the process safer and more precise.
"This invention is designed to further improve cryoablation's efficiency and to expand the number of indications we can deliver in the future," said Eyal Shamir, IceCure's Chief Executive Officer. "As we await the U.S. Food and Drug Administration's ("FDA") decision on marketing approval for ProSense® in early-stage breast cancer when combined with adjuvant endocrine therapy for women aged 70 and over, expanding our intellectual property assets around our platform cryoablation technology is more important than ever."
The patent protects this innovation until 2045 and could support expanded use in treating various cancers.
Source: https://usanewsgroup.com/2024/09/21/is-oncolytics-biotech-the-markets-most-undervalued-cancer-opportunity/
CONTACT:
Baystreet.ca
[email protected]
(250) 999-4849
DISCLAIMER: Nothing in this publication should be considered as personalized financial advice. We are not licensed under securities laws to address your particular financial situation. No communication by our employees to you should be deemed as personalized financial advice. Please consult a licensed financial advisor before making any investment decision. This is a paid advertisement and is neither an offer nor recommendation to buy or sell any security. We hold no investment licenses and are thus neither licensed nor qualified to provide investment advice. The content in this report or email is not provided to any individual with a view toward their individual circumstances. Baystreet.ca is a wholly-owned subsidiary of Baystreet.ca Media Corp. (“BAY”) BAY has been not been paid a fee for Oncolytics Biotech Inc. advertising and/or digital media, but the owner(s) of BAY also own Market IQ Media Group, Inc., which has been paid a fee from the company directly. There may be 3rd parties who may have shares Oncolytics Biotech Inc., and may liquidate their shares which could have a negative effect on the price of the stock. This compensation constitutes a conflict of interest as to our ability to remain objective in our communication regarding the profiled company. Because of this conflict, individuals are strongly encouraged to not use this publication as the basis for any investment decision. The owner/operator of BAY own shares of Oncolytics Biotech Inc. which were purchased in the open market. BAY and all of it’s respective employees, owners and affiliates reserve the right to buy and sell, and will buy and sell shares of Oncolytics Biotech Inc. at any time thereafter without any further notice. We also expect further compensation as an ongoing digital media effort to increase visibility for the company, no further notice will be given, but let this disclaimer serve as notice that all material disseminated by BAY has been approved by the above mentioned company; this is a paid advertisement, and we own shares of the mentioned company that we will sell, and we also reserve the right to buy shares of the company in the open market, or through other investment vehicles. While all information is believed to be reliable, it is not guaranteed by us to be accurate. Individuals should assume that all information contained in our newsletter is not trustworthy unless verified by their own independent research. Also, because events and circumstances frequently do not occur as expected, there will likely be differences between any predictions and actual results. Always consult a licensed investment professional before making any investment decision. Be extremely careful, investing in securities carries a high degree of risk; you may likely lose some or all of the investment.